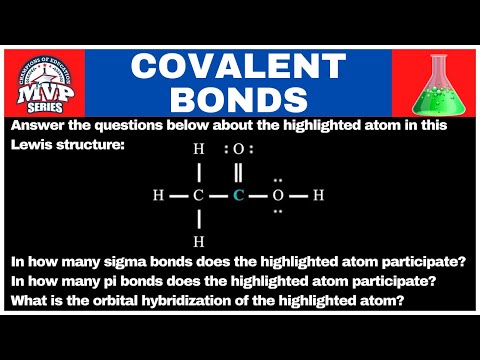
Determine the limiting reactant and the mass of nitrogen that can be formed from 50 g N2O4
Determine the limiting reactant (LR) and the mass (in g) of nitrogen that can be formed from 50.0 g N2O4 and 45.0 g N2H4. Some possibly useful molar masses are as follows: N2O4 = 92.02 g/mol, N2H4 = 32.05 g/mol.