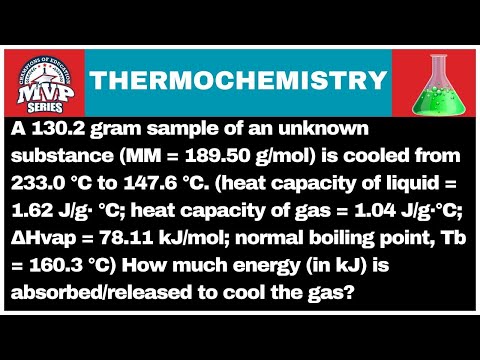
A chemist must prepare 575. mL of 203. μM aqueous magnesium fluoride (MgF2) working solution. She'll do this by pouring out some 549
A chemist must prepare 575. mL of 203. μM aqueous magnesium fluoride (MgF2) working solution. She'll do this by pouring out some 549. μmol/L aqueous magnesium fluoride stock solution into a graduated cylinder and diluting it with distilled water.